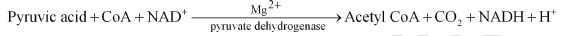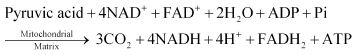Micrographia was written by Robert hooke
The secrets of nature was written by Leeuwenhoek
Showing posts with label Biology. Show all posts
Showing posts with label Biology. Show all posts
22 May 2020
15 Apr 2019
Respiration in Plants
Chapter 14 : Respiration in Plants

Cellular Respiration
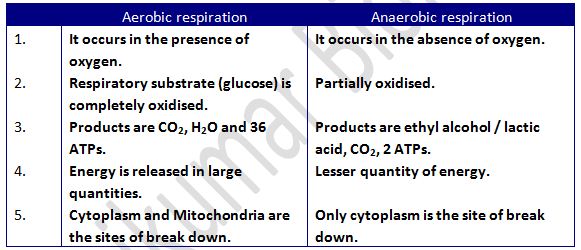
· Aerobic respiration
· Anaerobic respiration
- It is the process of oxidation / breakdown of food materials within the cell to release energy. Respiratory substrate to be oxidised during respiration is usually glucose, but these can also be proteins, fats or organic acids.
- In plants respiration gas exchange occurs through stomata and lenticels.
- Overall cellular respiration is:
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy (36 ATPs)
· Aerobic respiration
· Anaerobic respiration
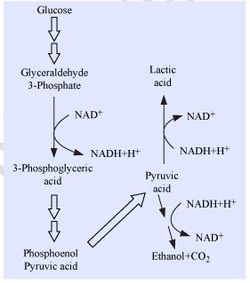
Mechanism of respiration :
· Glycolysis – it is common to both aerobic and anaerobic respiration
· Citric acid cycle / Krebs cycle - Aerobic respiration in mitochondria
· Electron transport system – in the inner membrane of mitochondria
· Both aerobic and anaerobic respiration starts with Glycolysis.
· In aerobic respiration Glycolysis is followed by Citric acid cycle and ETS (both occur in mitochondria).
· In anaerobic respiration Glycolysis is followed by formation of ethyl alcohol / lactic acid in the cytoplasm.
Fermentation :
Incomplete oxidation of pyruvic acid, under anaerobic respiration forms lactic acid/ ethyl alcohol. It occurs in bacteria, yeast and striated muscles.
In yeast fermentation:
o Pyruvic acid → Ethanol + CO2
o Enzymes involved − Pyruvic acid decarboxylase, Alcohol dehydrogenas.
o Pyruvic acid → Lactic acid.
o Enzyme involved − Lactate dehydrogenase.
o While doing severe exercise similar reaction occurs in animal muscles in anaerobic conditions.
· Glycolysis – it is common to both aerobic and anaerobic respiration
· Citric acid cycle / Krebs cycle - Aerobic respiration in mitochondria
· Electron transport system – in the inner membrane of mitochondria
· Both aerobic and anaerobic respiration starts with Glycolysis.
· In aerobic respiration Glycolysis is followed by Citric acid cycle and ETS (both occur in mitochondria).
· In anaerobic respiration Glycolysis is followed by formation of ethyl alcohol / lactic acid in the cytoplasm.
Fermentation :
Incomplete oxidation of pyruvic acid, under anaerobic respiration forms lactic acid/ ethyl alcohol. It occurs in bacteria, yeast and striated muscles.
In yeast fermentation:
o Pyruvic acid → Ethanol + CO2
o Enzymes involved − Pyruvic acid decarboxylase, Alcohol dehydrogenas.
- Only 7% of energy of glucose is released during fermentation.
- Yeasts poison themselves to death when alcohol concentration reaches about 13%.
o Pyruvic acid → Lactic acid.
o Enzyme involved − Lactate dehydrogenase.
o While doing severe exercise similar reaction occurs in animal muscles in anaerobic conditions.
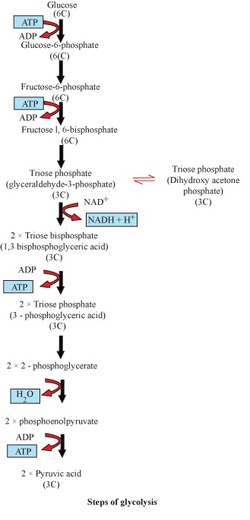
Glycolysis :
- It is the process of breaking down of glucose to pyruvic acid.
- It was given by Embden, Meyerhof and Parnas
- A chain of 10 reactions converts glucose into pyruvate.
- Net ATPs produced = 4 (produced) − 2 (consumed) = 2 ATPs
- The pyruvate, so produced, may undergo:
- Lactic acid fermentation
- Alcoholic fermentation
- Aerobic respiration (Krebs cycle)
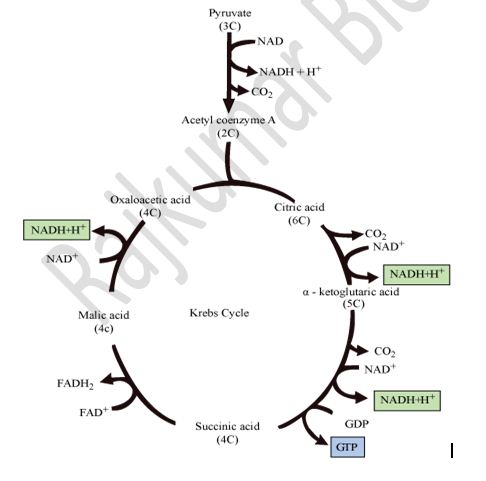
Citric acid cycle / Tricarboxylic acid cycle / Kreb’s cycle:
- TCA cycle – it takes place in the mitochondrial matrix – it is the process of complete oxidation of pyruvate by stepwise removal of all hydrogen atoms, which leaves three molecules of CO2
- Electron Transport Chain and Oxidative phosphorylation – it takes place in the inner membrane of the mitochondria – it is the process of synthesis of ATP fron NADH2 and FADH2.
Formation of Acetyl Coenzyme A
Krebs Cycle/ Tricarboxylic acid cycle / Citric acid cycle:
Overall equation
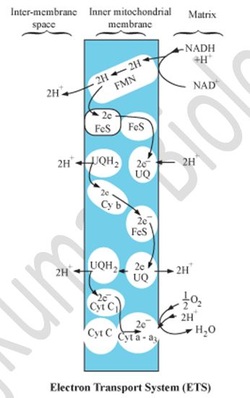
Electron Transport Chain (ETS)
- NADH2 and FADH2 are oxidised to release the energy stored in them in the form of ATPs.
- Electrons are passed from one carrier to another, and finally to oxygen, resulting in the formation of water.
- Oxidation of 1 NADH produces 3 ATPs.
Oxidation of 1 FADH2 produces 2 ATPs.
Oxidative Phosphorylation

Respiratory Balance Sheet
Respiratory Quotient (RQ)
· RQ is less than 1 for fats.
2 C51 H98 O6 +145 O2 - --> 102CO2 + 98H2O + Energy
RQ = 102 CO2
-------------- = 0.7
145 O2
· RQ is 0.9 for proteins.
· RQ is more than 1 for organic acids.
· RQ is infinite in case of anaerobic resp. because CO2 is evolved but O2 is not consumed
- Glucose + 6O2 + 36ADP + 36Pi → 6CO2 + 6H2O + 36ATP
Respiratory Quotient (RQ)
- It is the ratio of the volume of CO2 evolved to the volume of O2 consumed during respiration.
- RQ = 1 (When carbohydrate is used as substrate)
· RQ is less than 1 for fats.
2 C51 H98 O6 +145 O2 - --> 102CO2 + 98H2O + Energy
RQ = 102 CO2
-------------- = 0.7
145 O2
· RQ is 0.9 for proteins.
· RQ is more than 1 for organic acids.
· RQ is infinite in case of anaerobic resp. because CO2 is evolved but O2 is not consumed
23 Jun 2017
Hypotheses about the origins of life
Hypotheses about the origins of life
The Oparin-Haldane hypothesis, Miller-Urey experiment, and RNA world.
Key points:
Q-1 WHEN DID EARTH FORMED?
- The Earth formed roughly 4.54, point, 5 billion years ago, and life probably began between 3.53, point, 5 and 3.93, point, 9 billion years ago.
- The Oparin-Haldane hypothesis suggests that life arose gradually from inorganic molecules, with “building blocks” like amino acids forming first and then combining to make complex polymers.
- The Miller-Urey experiment provided the first evidence that organic molecules needed for life could be formed from inorganic components.
- Some scientists support the RNA world hypothesis, which suggests that the first life was self-replicating RNA. Others favor the metabolism-first hypothesis, placing metabolic networks before DNA or RNA.
- Simple organic compounds might have come to early Earth on meteorites.
Introduction
Q-2 If there were other life out there in the universe, how similar do you think it would it be to life on Earth?
Q-3 Would it use DNA as its genetic material, like you and me? Would it even be made up of cells?
- We can only speculate about these questions, since we haven't yet found any life forms that hail from off of Earth.
- But we can think in a more informed way about whether life might exist on other planets (and under what conditions) by considering how life may have arisen right here on our own planet.
- In this article, we'll examine scientific ideas about the origin of life on Earth.
The when of life's origins (3.53, point, 5 billion years ago or more) is well-supported by fossils and radiometric dating.
- But the how is much less understood. In comparison to the central dogma or the theory of evolution, hypotheses about life's origins are much more...hypothetical.
- No one is sure which hypothesis is correct – or if the correct hypothesis is still out there, waiting to be discovered.
When did life appear on Earth?
Geologists estimate that the Earth formed around 4.54, point, 5 billion years ago. This estimate comes from measuring the ages of the oldest rocks on Earth, as well the ages of moon rocks and meteorites, by radiometric dating (in which decay of radioactive isotopes is used to calculate the time since a rock’s formation).
For many millions of years, early Earth was pummeled by asteroids and other celestial objects. Temperatures also would have been very high (with water taking the form of a gas, not a liquid). The first life might have emerged during a break in the asteroid bombardment, between 4.44, point, 4 and 4.04, point, 0 billion years ago, when it was cool enough for water to condense into oceans1start superscript, 1, end superscript. However, a second bombardment happened about 3.93, point, 9 billion years ago. It’s likely after this final go-round that Earth became capable of supporting sustained life.
The earliest fossil evidence of life
The earliest evidence of life on Earth comes from fossils discovered in Western Australia that date back to about 3.53, point, 5 billion years ago. These fossils are of structures known as stromatolites, which are, in many cases, formed by the growth of layer upon layer of single-celled microbes, such as cyanobacteria. (Stromatolites are also made by present-day microbes, not just prehistoric ones.)

Image credit: "Stromatolite," by Didier Descouens, CC BY-SA 4.0.
The earliest fossils of microbes themselves, rather than just their by-products, preserve the remains of what scientists think are sulfur-metabolizing bacteria. The fossils also come from Australia and date to about 3.43, point, 4 billion years ago2start superscript, 2, end superscript.
Bacteria are relatively complex, suggesting that life probably began a good deal earlier than 3.53, point, 5 billion years ago. However, the lack of earlier fossil evidence makes pinpointing the time of life’s origin difficult (if not impossible).
How might life have arisen?
In the 1920s, Russian scientist Aleksandr Oparin and English scientist J. B. S. Haldane both separately proposed what's now called the Oparin-Haldane hypothesis: that life on Earth could have arisen step-by-step from non-living matter through a process of “gradual chemical evolution.” 3start superscript, 3, end superscript
Oparin and Haldane thought that the early Earth had a reducing atmosphere, meaning an oxygen-poor atmosphere in which molecules tend to donate electrons. Under these conditions, they suggested that:
- Simple inorganic molecules could have reacted (with energy from lightning or the sun) to form building blocks like amino acids and nucleotides, which could have accumulated in the oceans, making a "primordial soup." 3start superscript, 3, end superscript
- The building blocks could have combined in further reactions, forming larger, more complex molecules (polymers) like proteins and nucleic acids, perhaps in pools at the water's edge.
- The polymers could have assembled into units or structures that were capable of sustaining and replicating themselves. Oparin thought these might have been “colonies” of proteins clustered together to carry out metabolism, while Haldane suggested that macromolecules became enclosed in membranes to make cell-like structures4,5start superscript, 4, comma, 5, end superscript.
The details of this model are probably not quite correct. For instance, geologists now think the early atmosphere was not reducing, and it's unclear whether pools at the edge of the ocean are a likely site for life's first appearance. But the basic idea – a stepwise, spontaneous formation of simple, then more complex, then self-sustaining biological molecules or assemblies – is still at the core of most origins-of-life hypotheses today.
From inorganic compounds to building blocks
In 1953, Stanley Miller and Harold Urey did an experiment to test Oparin and Haldane’s ideas. They found that organic molecules could be spontaneously produced under reducing conditions thought to resemble those of early Earth.
Miller and Urey built a closed system containing a heated pool of water and a mixture of gases that were thought to be abundant in the atmosphere of early earth (H2H, start subscript, 2, end subscriptOO, NH4N, H, start subscript, 4, end subscript, CH4C, H, start subscript, 4, end subscript, and N2N, start subscript, 2, end subscript). To simulate the lightning that might have provided energy for chemical reactions in Earth’s early atmosphere, Miller and Urey sent sparks of electricity through their experimental system.

Cartoon depiction of the apparatus used by Miller and Urey to simulate conditions on early Earth.
Image credit: "Miller and Urey's experiment," by CK-12 Foundation, CC BY-NC 3.0.
After letting the experiment run for a week, Miller and Urey found that various types of amino acids, sugars, lipids and other organic molecules had formed. Large, complex molecules like DNA and protein were missing, but the Miller-Urey experiment showed that at least some of the building blocks for these molecules could form spontaneously from simple compounds.
Were Miller and Urey's results meaningful?
Scientists now think that the atmosphere of early Earth was different than in Miller and Urey's setup (that is, not reducing, and not rich in ammonia and methane)6,7start superscript, 6, comma, 7, end superscript. So, it's doubtful that Miller and Urey did an accurate simulation of conditions on early Earth.
However, a variety of experiments done in the years since have shown that organic building blocks (especially amino acids) can form from inorganic precursors under a fairly wide range of conditions8start superscript, 8, end superscript.
start superscript, 9, end superscript
From these experiments, it seems reasonable to imagine that at least some of life's building blocks could have formed abiotically on early Earth. However, exactly how (and under what conditions) remains an open question.
From building blocks to polymers
How could monomers (building blocks) like amino acids or nucleotides have assembled into polymers, or actual biological macromolecules, on early Earth? In cells today, polymers are put together by enzymes. But, since the enzymes themselves are polymers, this is kind of a chicken-and-egg problem!
Monomers may have been able to spontaneously form polymers under the conditions found on early Earth. For instance, in the 1950s, biochemist Sidney Fox and his colleagues found that if amino acids were heated in the absence of water, they could link together to form proteins10start superscript, 10, end superscript. Fox suggested that, on early Earth, ocean water carrying amino acids could have splashed onto a hot surface like a lava flow, boiling away the water and leaving behind a protein.

Image credit: "Kusový montmorillonit," by Jan Kameníček, CC BY-SA 3.0.
Additional experiments in the 1990s showed that RNA nucleotides can be linked together when they are exposed to a clay surface11start superscript, 11, end superscript. The clay acts as a catalyst to form an RNA polymer. More broadly, clay and other mineral surfaces may have played a key role in the formation of polymers, acting as supports or catalysts. Polymers floating in solution might have hydrolyzed (broken down) quickly, supporting a surface-attached model12start superscript, 12, end superscript.
The image above shows a sample of a type of clay known as montmorillonite. Montmorillonite in particular has catalytic and organizing properties that may have been important in the origins of life, such as the ability to catalyze formation of RNA polymers (and also the assembly of cell-like lipid vesicles)13start superscript, 13, end superscript.
What was the nature of the earliest life?
If we imagine that polymers were able to form on early Earth, this still leaves us with the question of how the polymers would have become self-replicating or self-perpetuating, meeting the most basic criteria for life. This is an area in which there are many ideas, but little certainty about the correct answer.
The "genes-first" hypothesis
One possibility is that the first life forms were self-replicating nucleic acids, such as RNA or DNA, and that other elements (like metabolic networks) were a later add-on to this basic system. This is called the genes-first hypothesis14start superscript, 14, end superscript.
Many scientists who subscribe to this hypothesis think that RNA, not DNA, was likely the first genetic material. This is known as the RNA world hypothesis. Scientists favor RNA over DNA as the first genetic molecule for several reasons. Perhaps the most important is that RNA can, in addition to carrying information, act as a catalyst. In contrast, we don’t know of any naturally occurring catalytic DNA molecules15,16start superscript, 15, comma, 16, end superscript.
RNA catalysts are called ribozymes, and they could have played key roles in the RNA world. A catalytic RNA could, potentially, catalyze a chemical reaction to copy itself. Such a self-replicating RNA could pass genetic material from generation to generation, fulfilling the most basic criteria for life and, potentially, undergoing evolution. In fact, researchers have been able to synthetically engineer small ribozymes that are capable of self-replication.
It’s also possible that RNA wasn’t the first information-carrying molecule to serve as genetic material. Some scientists think that an even simpler “RNA-like” molecule with catalytic and information-carrying capacity might have come first, and might have catalyzed or acted as a template for RNA synthesis. This is sometimes called the "pre-RNA world" hypothesis17start superscript, 17, end superscript.
The "metabolism-first" hypothesis
An alternative to the genes-first hypothesis is the metabolism-first hypothesis, which suggests that self-sustaining networks of metabolic reactions may have been the first simple life (predating nucleic acids)14,18start superscript, 14, comma, 18, end superscript.
These networks might have formed, for instance, near undersea hydrothermal vents that provided a continual supply of chemical precursors, and might have been self-sustaining and persistent (meeting the basic criteria for life). In this scenario, initially simple pathways might have produced molecules that acted as catalysts for the formation of more complex molecules18start superscript, 18, end superscript. Eventually, the metabolic networks might have been able to build large molecules such as proteins and nucleic acids. Formation of "individuals" enclosed by membranes (separate from the communal network) would have been a late step14start superscript, 14, end superscript.
What might early cells have looked like?
A basic property of a cell is the ability to maintain an internal environment different from the surrounding environment. Today’s cells are separated from the environment by a phospholipid bilayer. It’s unlikely that phospholipids would have been present under the conditions in which the first cells formed, but other types of lipids (ones that would have more likely been available) have also been shown to spontaneously form bilayered compartments19start superscript, 19, end superscript.
In principle, this type of compartment could surround a self-replicating ribozyme or the components of a metabolic pathway, making a very basic cell. Though intriguing, this type of idea is not yet supported by experimental evidence – i.e., no experiment has yet been able to spontaneously generate a self-replicating cell from abiotic (non-living) components.
Another possibility: Organic molecules from outer space
Organic molecules might have formed spontaneously from inorganic ones on early Earth, à la Miller-Urey. But could they instead have come from space?
The idea that organic molecules might have traveled to Earth on meteorites may sound like science fiction, but it's supported by reasonable evidence. For example, scientists have found that organic molecules can be produced from simple chemical precursors present in space, under conditions that could exist in space (high UV irradiation and low temperature)20start superscript, 20, end superscript. We also know that some organic compounds are found in space and in other star systems.
Most importantly, various meteorites have turned out to contain organic compounds (derived from space, not from Earth). One meteorite, ALH84001, came from Mars and contained organic molecules with multiple ring structures. Another meteorite, the Murchison meteorite, carried nitrogenous bases (like those found in DNA and RNA), as well as a wide variety of amino acids.

Image credit: "ALH84001 meteorite, on display at Smithsonian Museum of Natural History," by J. L. Stuby, CC BY-SA 3.0.
One meteorite that fell in 2000 in Canada contained tiny organic structures dubbed "organic globules." NASA scientists think this type of meteorite might have fallen to Earth often during the planet's early history, seeding it with organic compounds21start superscript, 21, end superscript.
Summary
How life originated on our planet is both a fascinating and incredibly complex question. We know roughly when life began, but how remains a mystery.
- Miller, Urey, and others showed that simple inorganic molecules could combine to form the organic building blocks required for life as we know it.
- Once formed, these building blocks could have come together to form polymers such as proteins or RNA.
- Many scientists favor the RNA world hypothesis, in which RNA, not DNA, was the first genetic molecule of life on Earth. Other ideas include the pre-RNA world hypothesis and the metabolism-first hypothesis.
- Organic compounds could have been delivered to early Earth by meteorites and other celestial objects.
These are not the only scientific ideas about how life might have originated, nor are any of them conclusive. Keep your ears (and your mind) open as new information becomes available and new scientific ideas are proposed concerning life's origins.
Subscribe to:
Comments (Atom)